Abstract
Introduction: Data continue to accumulate showing older adults with hematologic malignancies, such as AML and MDS, derive a survival benefit from HCT. However, as HCT expands into older and less fit patients (pts), the inability to describe and predict post-HCT toxicity presents a major barrier to appropriate and broader application. Standard endpoints of non-relapse mortality (NRM), organ toxicity, graft-versus host disease (GvHD), or overall survival (OS) miss important common geriatric complications (e.g., delirium, disability, falls). We aimed to estimate disability-free survival (DiFS), a composite endpoint of death, dementia or physical disability, used in older adult non-oncology trials (McNeil J, N Engl J Med 2018; 379; 1499-1508) by summarizing disability in the early post-HCT context.
Methods: We reviewed electronic records for the development of disability through day 100 for pts ≥ 60 years undergoing first HCT from 2013 - 2019 at City of Hope National Medical Center. Disability events included delirium affecting instrumental ADLs (i.e., CTC ≥ 2), falls, ICU admission, or needing mobility assistance. Mobility assistance leveraged a standardized nursing flowsheet tool completed every shift for hospitalized pts, comprised of 5 categories of ambulation independence (independent, assistive equipment, assistive person, assistive equipment and person, complete dependent); mobility timepoints captured were pre-HCT, weekly starting on day 0 to d21 and at hospital discharge. Categorization of assistive person or more after HCT met the mobility assistance requirement. All pts required hospitalization post-HCT in this era. Failure events for DiFS included first disability event as above or death. Kaplan-Meier method was used to calculate DiFS at d30 and d100. A conditional landmark analysis examined the differences in unadjusted NRM among pts experiencing disability prior to d30 or d100 landmark dates and alive at the landmark date.
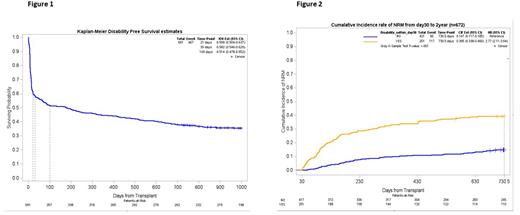
Results: Among 699 pts, 19.7% were ≥ 70 years, 77.0% had AML/MDS/MPN, 95.6% received reduced intensity conditioning (RIC), 80.1% were conditioned with fludarabine/melphalan, and 86.7% received matched donors. Few pts needed mobility assistance pre-HCT (4.4%) although 28.3% developed mobility assistance by d21. The cumulative incidence of other disability events at d30 and d100 were: falls (4.9%, 14.3%), delirium (24.3%, 29.3%), and ICU admission (15.3%, 20.2%). NRM was 3.9% at d30 and 26.9% at 2 years. Figure 1 shows DiFS at d30 and d100 of 58.2% and 51.4%. The most common d30 disability events were delirium (41%) and mobility assistance (34.7%); all deaths by d30 (n=27) were preceded by a disability event. Baseline factors in univariate analysis associated with inferior d30 DiFS included KPS < 90% (p=.001), age ≥ 70 (.01), non-matched related donor (.003) but not HCT-CI ≥ 3, time period (<2018), conditioning regimen, GVHD prophylaxis, or baseline mobility assistance. D30 disability events were strongly linked to elevated 2 yr NRM by d30 landmark analysis (figure 2)(40% vs 15% without d30 disability events, HR=2.77; 95% CI 2.11-3.64). Similarly, disability by d100 negatively influenced d100 to 2 year NRM (HR =2.27, 95% CI: 1.65-3.11).
Conclusions: HCT among older adults engenders frequent geriatric complications of delirium, loss of ambulation independence, falls and ICU admission in the early HCT period. Consequently, DiFS is approximately 60% at day 30. Further, early disability strongly predicts for higher risk of future NRM. DiFS should be further explored as a novel endpoint to summarize functional loss after older adult HCT.
Disclosures
Aldoss:Amgen: Consultancy; Agios: Consultancy, Honoraria; Autolus Limited: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Kite: Consultancy; AbbVie: Consultancy, Research Funding. Ali:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Aribi:SeaGen: Consultancy. Pullarkat:AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member. Salhotra:Orca Bio: Research Funding; BMS: Research Funding; Kadmon: Other: Advisory board meeting . Stein:Amgen: Speakers Bureau. Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings. Al Malki:CareDx: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Research Funding; Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; NexImmune: Consultancy, Research Funding. Nakamura:Helocyte Inc: Research Funding; Omeros: Consultancy; BluebirdBio: Consultancy; Magenta Therapeutics: Consultancy; Sanofi: Consultancy; Kadmon: Consultancy. Artz:Abbvie: Honoraria; Magenta: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.